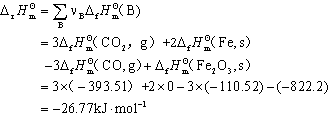2-10解:(1)×;(2)×;(3)√;(4)×;(5)×;(6)×;
(7)×;(8)×;(9);(10)×;(11)×
2-11解:(1)敞开体系;(2)孤立体系;(3)敞开体系;
2-12解:(1) Q =100kJ W=-500 kJ △U = Q + W=-400 kJ
(2)Q =-100kJ W=500 kJ △U = Q + W=400 k
2-13解:因为此过程为可逆相变过程,所以
Qp =△H= 40.6kJ·mol-1
W=-p外△V≈-nRT =-8.314×373.15=-3.10 kJ·mol-1
△U = Q + W= 40.6+(-3.10)=37.5 kJ·mol-1
2-14解:(1)